Management and Treatment
 Why is exocrine pancreatic insufficiency (EPI)
treatment important?
Why is exocrine pancreatic insufficiency (EPI)
treatment important?
If left untreated, EPI can cause:1,2
- Complications related to fat malabsorption and malnutrition
- Negative impact on quality of life
- Decreased overall survival
After an EPI diagnosis is established, a comprehensive EPI management plan consisting of the following should be initiated:1
Lifestyle
modifications
Avoiding alcohol, quitting smoking, and consuming frequent, small meals, with low to moderate fat. A very low‑fat diet should be avoided since it can cause fat‑soluble vitamin and essential fatty acid deficiencies.
Nutritional
supplementation
Nutritional supplementation, if deemed necessary during assessment, may include vitamins A, D, E, K, and B12, in addition to folate, magnesium, selenium zinc, iron, etc.
Medical
management
Pancreatic enzyme replacement therapy (PERT), a mixture of porcine-derived lipases, proteases, and amylases, is the standard of care for EPI treatment.
As part of the thorough care, treatment of underlying diseases that cause EPI is crucial.3
How Do PERTs Work?
The goal of medical management is to deliver sufficient pancreatic enzymatic activity into the duodenal lumen at the same time as the food to help restore nutrient digestion and aid absorption.
What are PERTs?
- PERT is the standard of care for treatment of EPI4
- PERT is a mixture of porcine-derived pancreatic enzymes and coenzymes including lipases, proteases, and amylases5
- FDA-approved PERT products are classified as pancrelipase due to the ratio and quantity of enzymes5
- Over-the-counter pancreatic enzymes are not regulated by the FDA and often their source of origin is not disclosed.
Mechanism of Action
Each of the following pancreatic enzymes in PERTs catalyze macronutrients to their building blocks in the duodenum and proximal small intestine, hence functioning like digestive enzymes that are secreted by the pancreas physiologically:5
Lipase
breaks down ester bonds of lipids
and fats to glycerol and fatty acids
Protease
breaks down proteins to
peptides and amino acids
Amylase
breaks down starches to dextrins
and short chain sugars
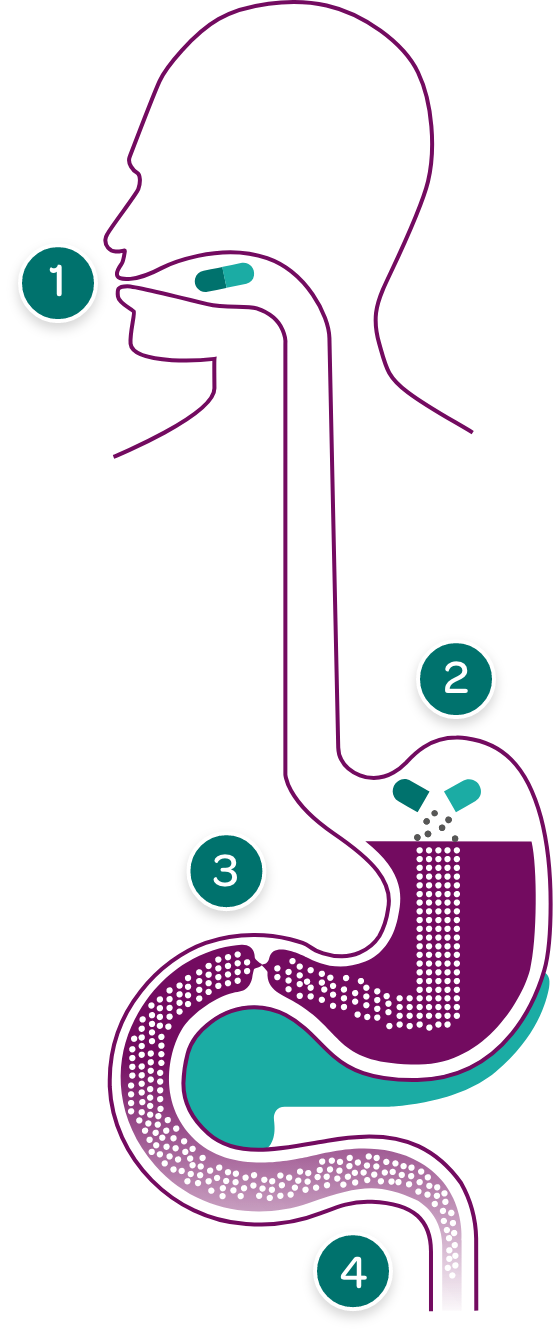
PERT capsules are taken with meals and snacks with sufficient fluid, so they can enter the stomach at the same time as food.
Enzyme complexes are small enough to pass from the stomach through the pylorus to the duodenum.
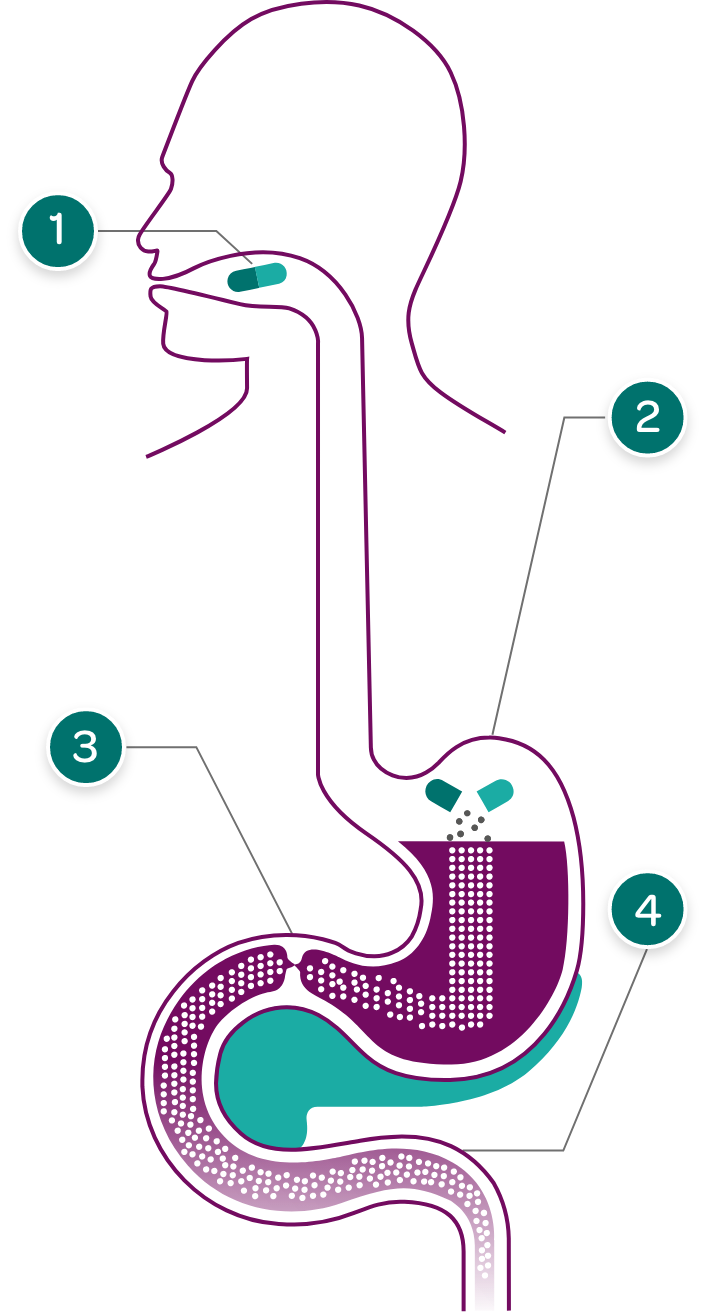
PERT capsules dissolve in the stomach releasing tiny pancreatic enzyme complexes.
Some PERTs have enteric coating to prevent digestion of the enzyme complexes in the stomach where pH is low and can deactivate enzyme activity.
In the duodenum and small intestine, pancreatic enzymes work to effectively break down fats, proteins, and carbohydrates in food. Enteric-coated PERTs are designed to dissolve in the duodenum in response to higher pH levels, thereby releasing the enzymes at the appropriate site of action.
PERT Dosing and Administration
The correct PERT dose is essential for effective EPI treatment
Dosing varies depending on the degree of maldigestion and fat content of the meal.
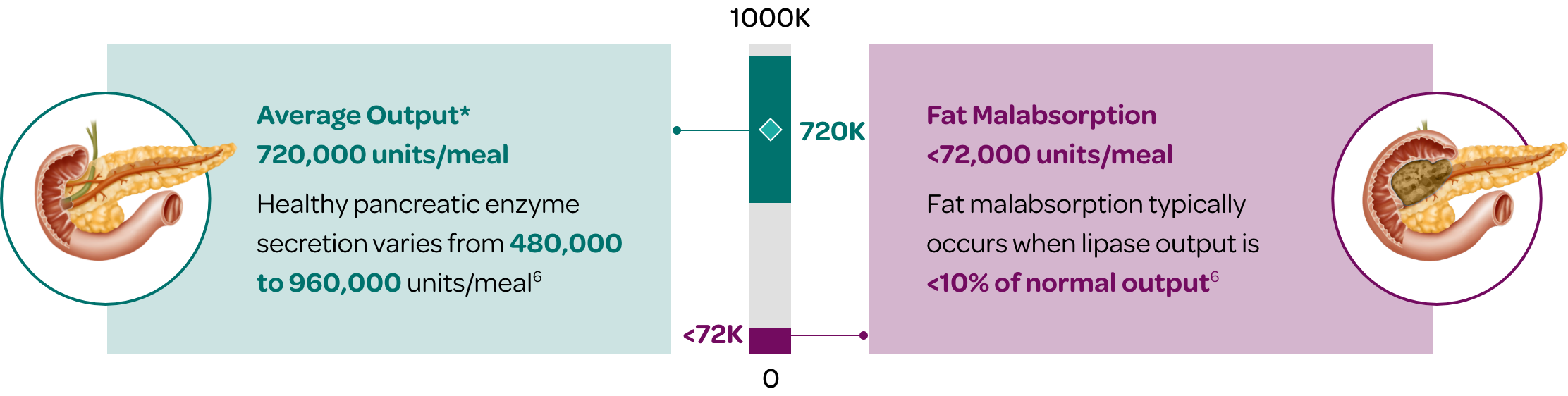
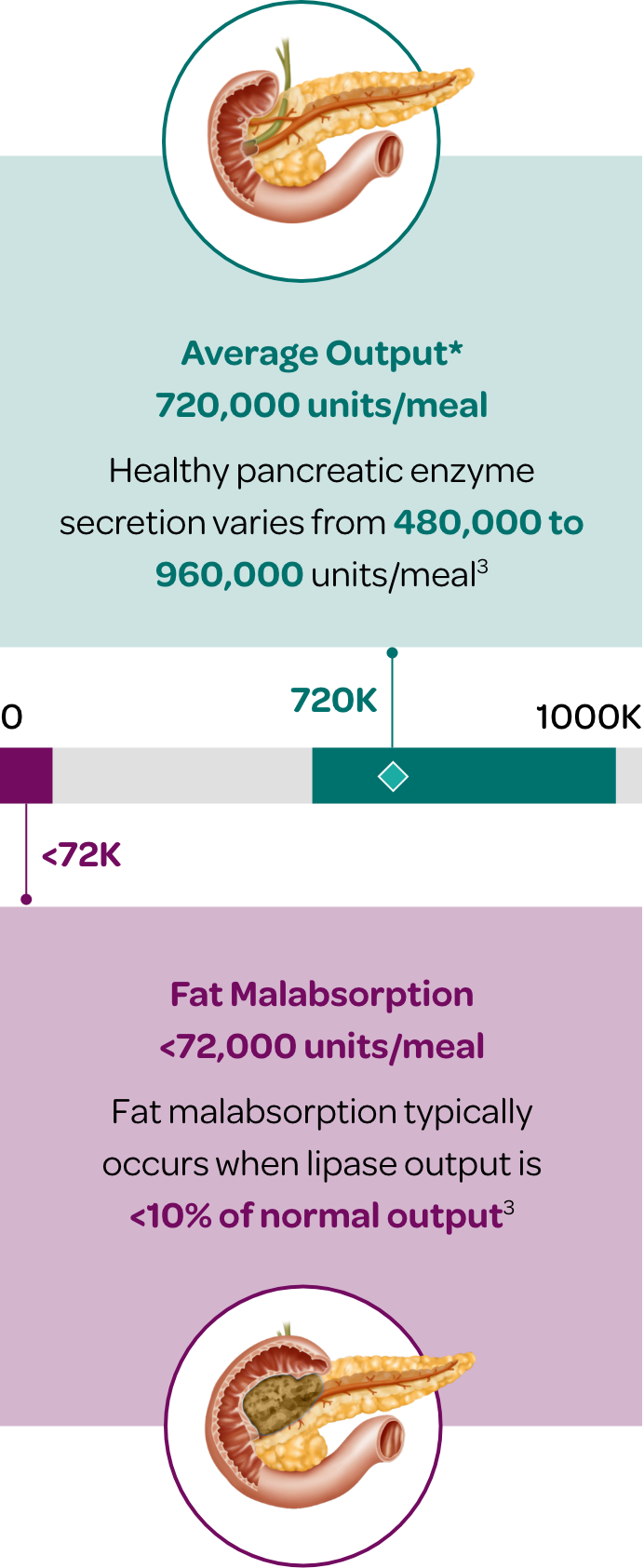
The healthy pancreas typically produces an average of 720,000 lipase units per meal (300-600kcal)6
- Normal pancreatic enzyme secretion varies with content and volume of meal ingested.
- Normal digestion also depends on postprandial synchrony between delivery of nutrients to the duodenum and discharge of pancreatic enzymes.
- ~10% of this prandial lipase output is required to maintain normal digestion.
- PERT is designed to mimic the exocrine secretory response of a healthy pancreas in terms of quantity, composition, and timing of luminal enzyme activity.
PERT dosing should be individualized. When prescribing a PERT:5
| Weight-Based Dosing5 | |
|---|---|
| Infants (up to 12 months) | 2,600—4,000 lipase units per 120 mL of formula or breastfeeding |
| Children >12 months and <4 years | 1,000 lipase units/kg/meal |
| Adults and children >4 years | 500 lipase units/kg/meal |
Please note that while the information provided is intended to be helpful, it is important to review the specific dosing for each PERT. Additional recommended doses, based on the underlying cause of EPI, may vary and should be followed as indicated on the individual PERT label.
Administration5
- PERT is orally administered
- PERT should be taken during meals or snacks with sufficient fluid
- PERT capsules/tablets should be swallowed whole
- PERT capsules/tablets should not be crushed, chewed, or retained in the mouth
- If a dose is missed, the next dose should be taken with the next meal or snack
General Safety of PERT
To avoid irritation of oral mucosa, patients should not chew PERT or retain in the mouth5
Exercise caution when administering PERT to a patient with a known allergy to proteins of porcine origin5
Exercise caution when prescribing PERT to patients with gout, renal impairment, or hyperuricemia5
Fibrosing colonopathy has been reported in patients with cystic fibrosis taking high-dose PERT5
Follow-up
Check in with patients about a week after initiating PERT to discuss symptoms and assess the need for a dose adjustment.4
During follow-up discuss and evaluate any changes in body weight, administration habits, and/or symptoms to determine if patient’s dose is appropriate or if dose adjustment is needed.
It is important to reassess treatment compliance and proper timing of doses in relation to meals, as lack of patient compliance and inadequate dosing are commonly observed in patients taking PERT.3
If signs and symptoms of malabsorption persist, increase the dosage. Titrate to either 2,500 lipase units/kg/meal, 10,000 lipase units/kg/day, or less than 4,000 lipase units/grams of fat ingested/day. Higher dosages may be administered if they are documented to be effective by fecal fat measures or an improvement in signs or symptoms of malabsorption including measures of nutritional status.5
Supplementation With Fat-Soluble Vitamins
Patients with EPI may require fat-soluble vitamin supplements simultaneously with PERT. Vitamin A, E, D, and K levels, as well as prothrombin time (PT)/international normalized ratio (INR), should be checked annually and 3 to 6 months after any vitamin therapy adjustment.1
What Are Treatment Challenges in EPI?
Inconsistencies exist among different providers in monitoring and managing EPI8
A retrospective cohort study evaluated FE-1 testing and PERT utilization across 3 provider types in patients diagnosed with chronic pancreatitis in an ambulatory clinic of a tertiary care hospital.
Percentage of Chronic Pancreatitis Patients Who Received
Adequate PERT Dosing by Type of Provider‡
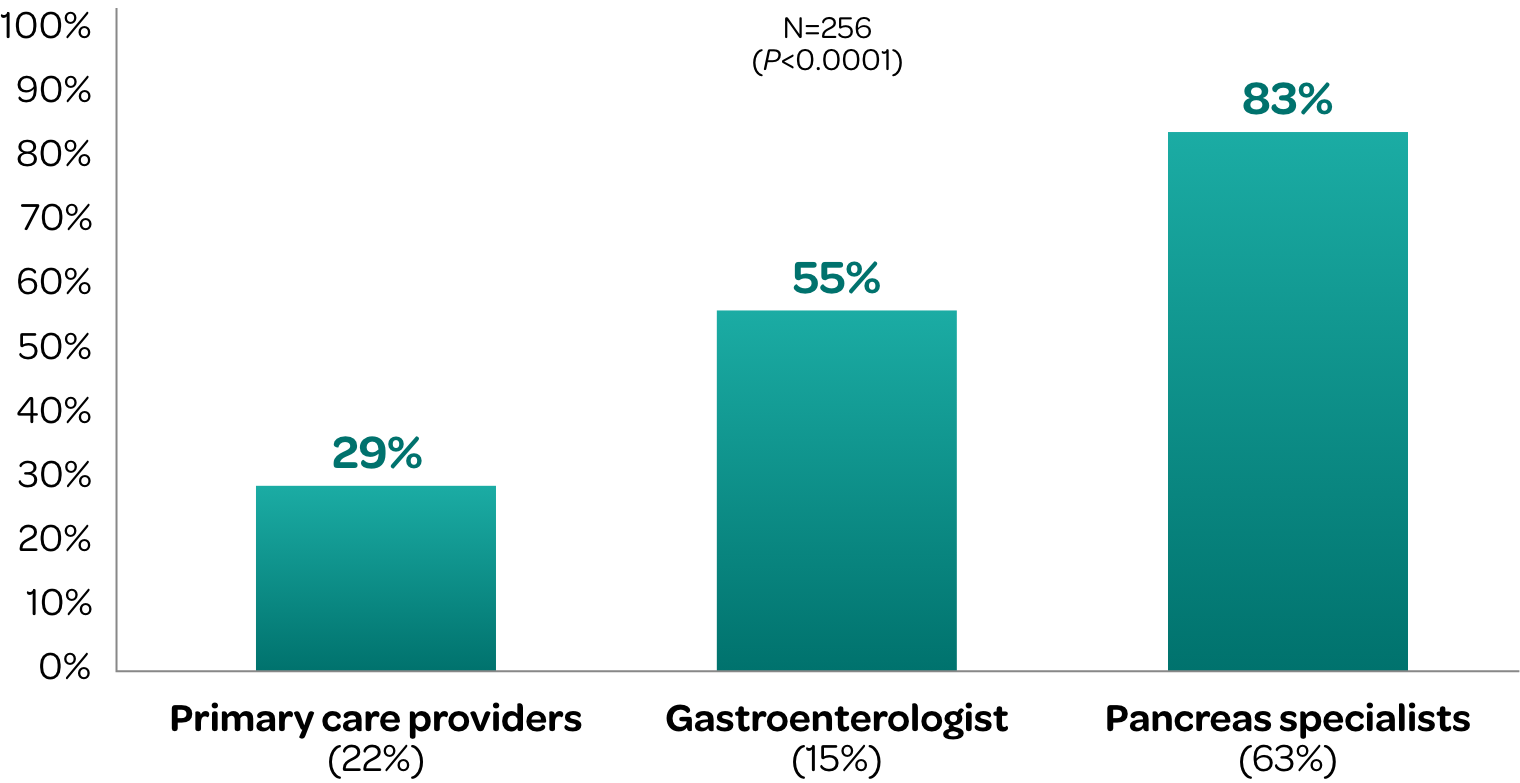
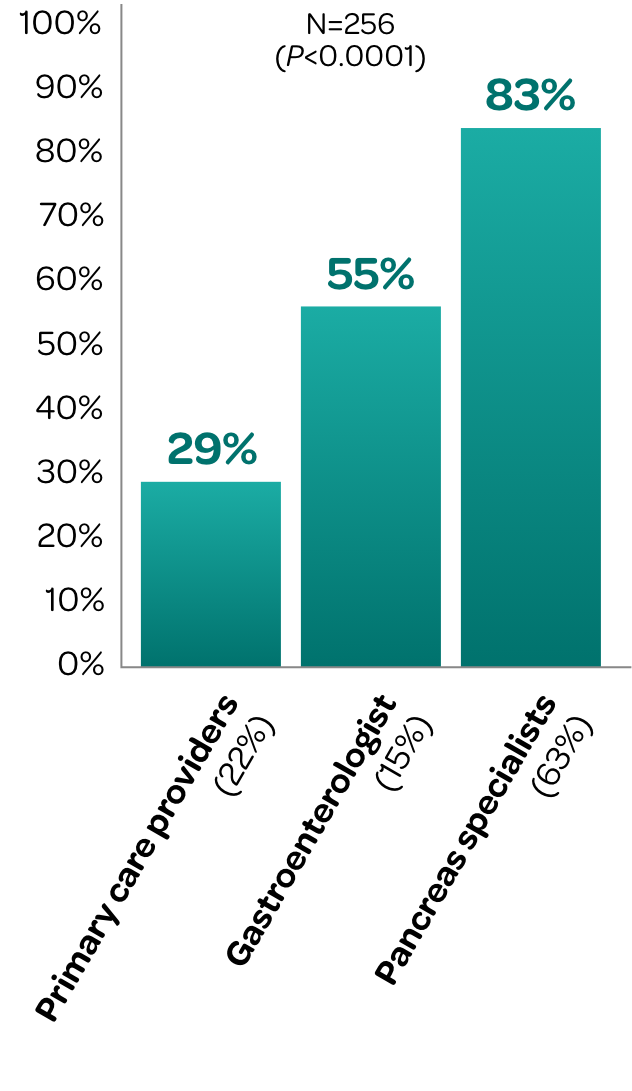
PERT dosing is often suboptimal
A retrospective study analysis of patients with a primary diagnosis of chronic pancreatitis or pancreatic cancer showed that:7
According to a survey of 75 patients with EPI or their caregivers:9
Many patients are dissatisfied with the advice they receive from their physician about PERT9,10
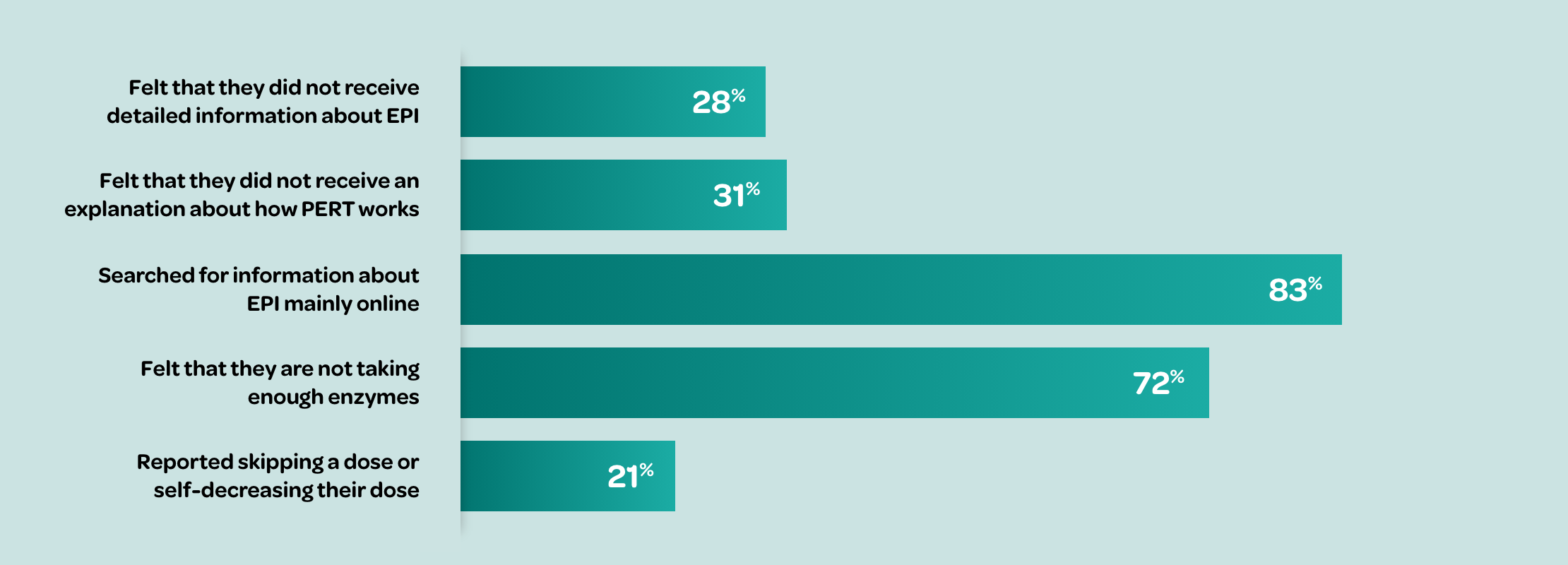
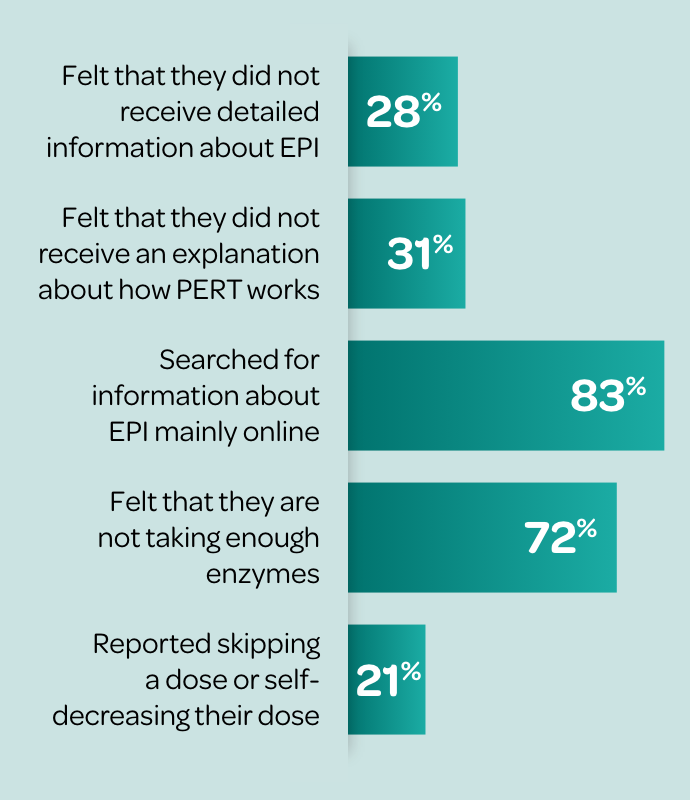
Many patients report that it takes ≥1 year to achieve optimal PERT dosing.10∥
According to a survey of 75 patients with EPI (or their caregivers):
~40% of patients reported absence of follow-up from their HCP since starting PERT9† despite recommendations for long-term monitoring11¶
~70% of patients stop taking PERT within 1 year12# due to inappropriate dosing, lack of education (e.g., dose timing), pill burden, and lack of provider follow-up9,10∥**

